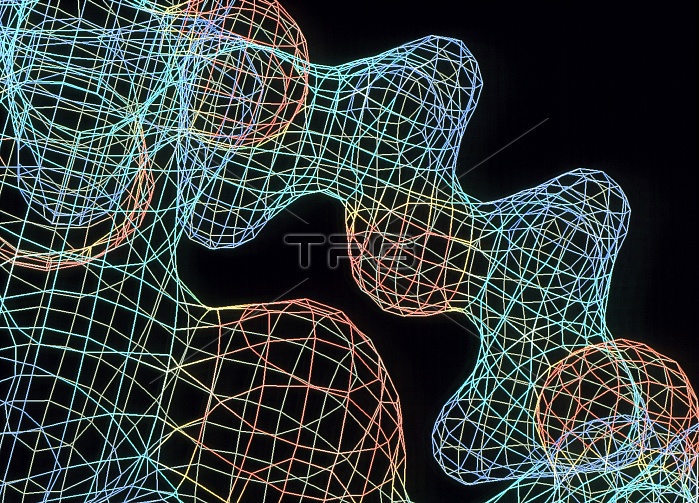
Crown ether. Wire-frame molecular graphic of part of the cyclic ether 18-crown-6 (molecular formula C12. H24. O6). The colour-coding of the surface represents the atoms beneath it: oxygen (red), carbon (light blue) and hydrogen (dark blue). The molecule is made up of six CH2. CH2. O groups linked in a ring. The naming of crown ethers arises from the total number of atoms in the ring (18) followed by the number of oxygen atoms (6). They are used to make ionic metal ions dissolve in non- ionic solvents, such as benzene. The ring's cavity size determines which ions it is best at capturing. 18-crown-6 is best at solvating potassium ions (K+), for example.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP10165647
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading