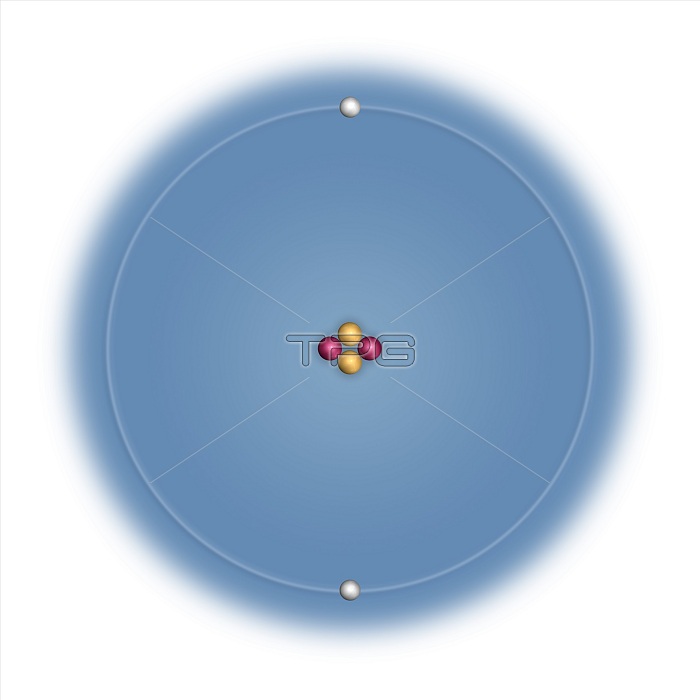
Helium (He). Diagram showing the nuclear composition and electron configuration of an atom of helium-4 (atomic number: 2), the most common isotope of the element helium. The nucleus consists of 2 protons (red) and 2 neutrons (yellow). Two electrons (white) bind to the nucleus, filling the first electron shell (ring) in what is a very stable configuration. The stability of an element's outer electrons determines its chemical and physical properties. Helium is a noble gas in group 18, period 1, and the s-block of the periodic table. At room temperature and pressure it is an inert gas that liquefies at minus 269 degrees Celsius.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP14864361
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
No
Right to Privacy:
No
Same folder images:

 Loading
Loading