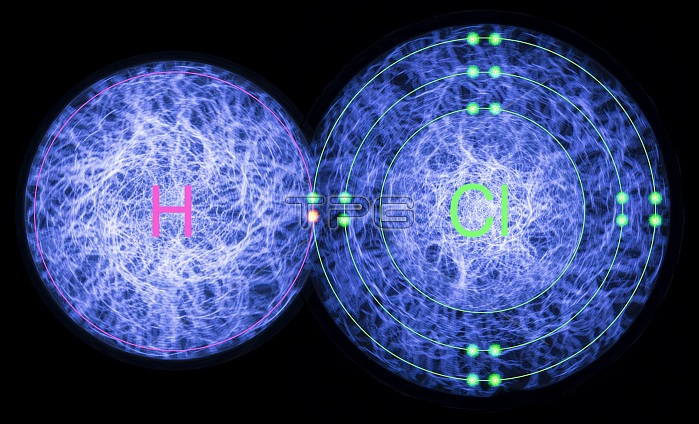
Hydrogen chloride. Composite image of the atomic and electronic structure of the molecule hydrogen chloride. This diatomic molecule consists of a hydrogen atom (H, left) forming a single covalent bond with a chlorine atom (Cl, right). This covalent bond is formed by the sharing of electrons (centre). The single hydrogen electron (pink) combines with the unpaired electron (green) in the chlorine atom's outer electron shell. This fills the outer electron shell, forming a strong and stable bond. Hydrogen chloride is a colourless gas that forms hydrochloric acid when dissolved in water.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP15983527
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading