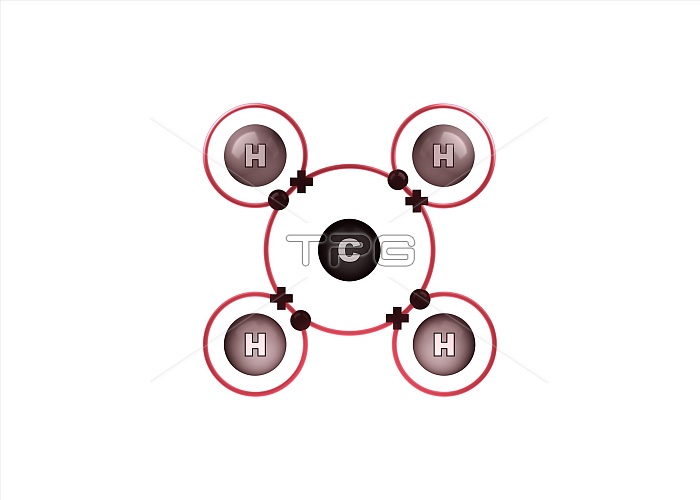
Bond formation in methane molecule. Illustration of the sharing of electrons (dots and crosses) between four hydrogen (H) and one carbon (C) atom to form a molecule of methane (CH4). This is an example of covalent bonding, with the four single bonds formed by a shared electron pair consisting of an electron from each atom. The outer electron shell of each atom is shown as a red ring. Methane is a gas at room temperature and pressure. For the atoms before the bonds form, see image C028/6470.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP16412215
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading