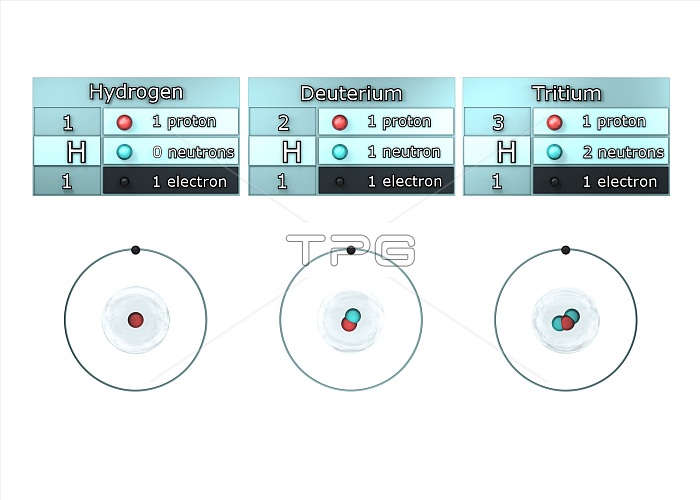
Isotopes of hydrogen. Illustration showing the isotopes of hydrogen. Isotopes are forms of an element that contain different numbers of neutrons in the atomic nucleus. Hydrogen-1, known as protium, has one proton (red) and no neutrons in its nucleus, hydrogen-2, deuterium, has one proton and one neutron (blue), and hydrogen-3, tritium, has one proton and two neutrons . All the isotopes also have one electron (black) orbiting the nucleus. Tritium is a radioactive isotope, but protium and deuterium are stable.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP16412217
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:
artworkatomatomicatomicstructurebohrchemicalchemistrycomparecomparisondeuteriumdeutrondiagramelectronelementheavyhydrogenhydrogenhydrogen2hydrogen3hydrogen-2hydrogen-3illustrationisotopeisotopicmodelneutronno-onenobodynucleusorbitparticlephysicalphysicsprotiumprotonradioactiverepresentationschematicstablestructuresubatomicparticlestablethreetriotritiumwhitebackground
23artworkatomatomicatomicbackgroundbohrchemicalchemistrycomparecomparisondeuteriumdeutrondiagramelectronelementheavyhydrogenhydrogenhydrogenhydrogenhydrogen-2hydrogen-3illustrationisotopeisotopicmodelneutronno-onenobodynucleusorbitparticleparticlesphysicalphysicsprotiumprotonradioactiverepresentationschematicstablestructurestructuresubatomictablethreetriotritiumwhite
 Loading
Loading