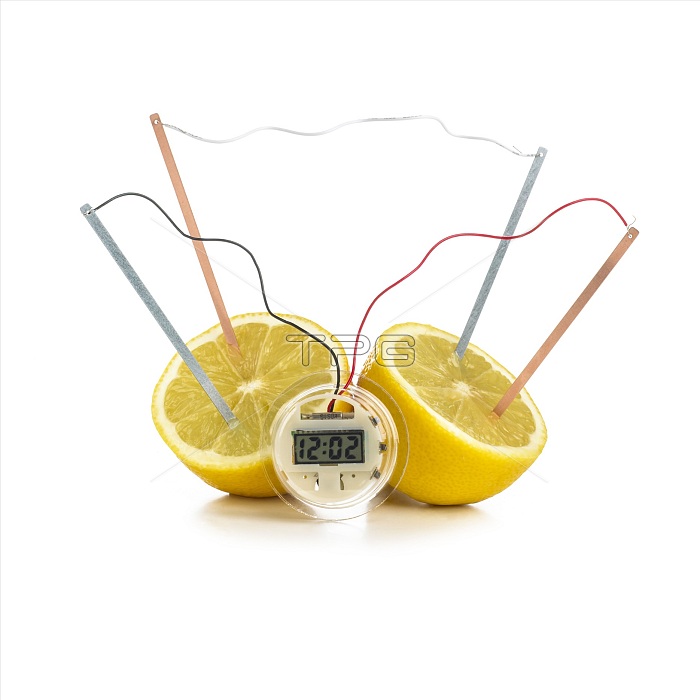
Lemon clock. Two halves of a lemon being used to provide electrical current sufficient to power an electronic clock. The lemon is being used as an electrolytic cell, the electrolyte being citric acid. Four electrodes - two copper and two zinc - are placed in the lemon and connected together. Zinc atoms on the electrode are oxidised, losing two electrons per atom and dissolving into solution. The electrons pass through the wires to the copper electrode where they combine with hydrogen ions to liberate hydrogen gas. The movement of electrons between electrodes form the current.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP16634072
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading