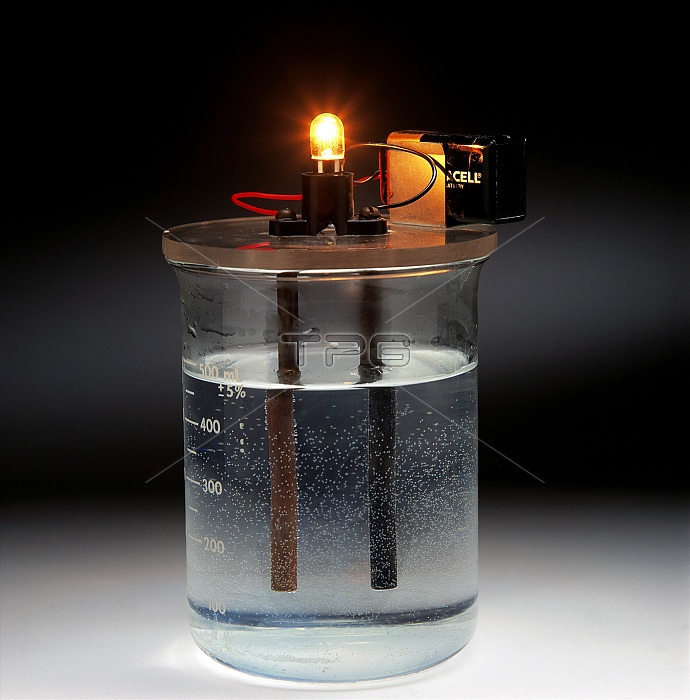
Conductivity. Demonstration of the electrical conductivity of potassium chloride (KCl) solution. The bulb is connected to a battery and two electrodes, forming an incomplete circuit. Because the solution conducts electricity, a current flows between the electrodes. This completes the circuit and allows the bulb to light. Pure water will not allow a current to flow. Potassium chloride is conductive because it is ionic: it splits into charged particles (ions) of K+ and Cl- in solution. It is these ions which allow the current to flow.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP22280784
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
No
Right to Privacy:
No
Same folder images:

 Loading
Loading