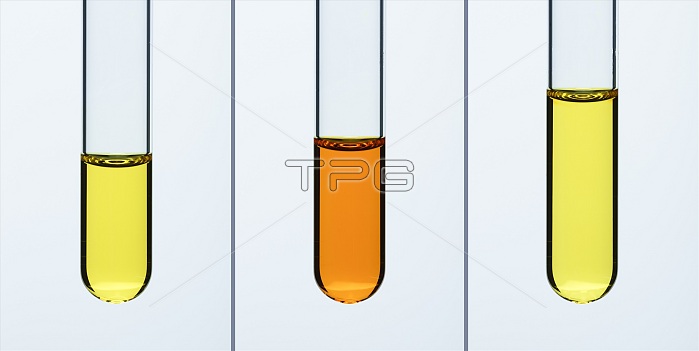
Chromate(VI)-dichromate(VI) equilibrium. Chromate(VI) ([CrO4]2-) and dichromate(VI) ([Cr2O7]2-) ions exist in an aqueous solution in equilibrium, according to the equation: [CrO4]2- + [H]+ <--> [Cr2O7]2- + H2O. According to Le Chatelier's Principle this equilibrium can be shifted by changing concentration of [H]+ ions (i.e. the pH of the solution). This is demonstrated in this series of photos. Left frame shows 4 mL of 0.25M solution of potassium chromate(VI) (K2CrO4, yellow). Middle frame was taken after 0.5 mL of 1M sulfuric acid (H2SO4) was added and the color changed to orange, indicating dichromate(VI) ions. Right frame was taken after 1.5 mL of 1M potassium hydroxide (KOH) was added and the color changed back to yellow indicating chromate(VI) ions.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP22304371
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
No
Right to Privacy:
No
Same folder images:

 Loading
Loading