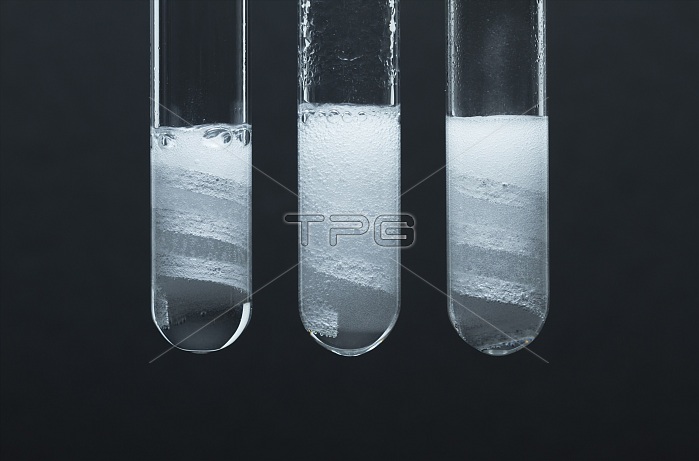
Reaction rate. Magnesium coils are placed in test tubes with left: concentrated (6M) acetic acid (CH3COOH); center: diluted (1M) hydrochloric acid (HCl); and right: diluted (1M) acetic acid. Magnesium reacts with the acids producing hydrogen bubbles: Mg + 2H+ -> Mg+ + H2. Hydrochloric acid is a strong acid and completely dissociates in solution. Acetic acid is a weak acid and only partially dissociates. The reaction proceeds with about the same rate in concentrated acetic acid and diluted hydrochloric acid (judged by the size and rate of hydrogen bubbles production). The reaction is slower in a diluted acetic acid.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP22305813
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
No
Right to Privacy:
No
Same folder images:

 Loading
Loading