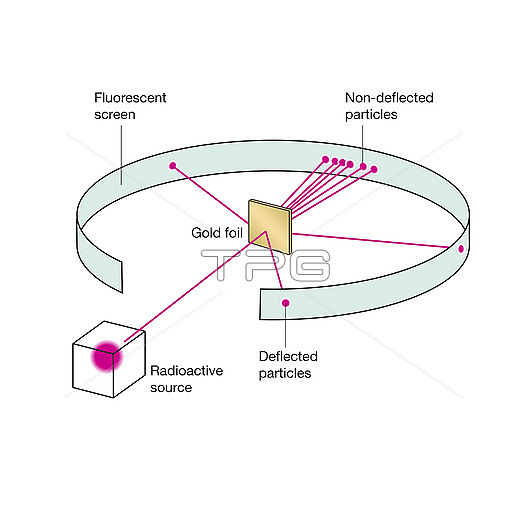
Rutherford scattering experiment. Illustration of the apparatus used to show the elastic scattering of charged particles according to Coulomb's law, which describes the electrostatic interaction between electrically charged particles, as demonstrated by Ernest Rutherford in 1911. Helium ions (alpha particles from a radiation source, pink) are fired at thin gold foil (centre) and the scattering pattern is observed on the ring-shaped surface. Most particles are deflected by only a small amount, but a few are deflected by large amounts, implying that the atomic nucleus is very small and positively charged. This phenomenon led to the development of the planetary Rutherford model of the atom and eventually the Bohr model.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP26152445
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading