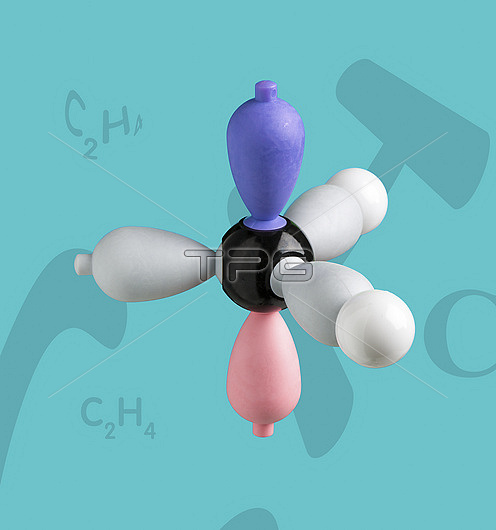
Electron density model of ethene before hybridisation Right hand side of Ethene model before hybridization. Ethene is a very important hydrocarbon which has the formula C2H4. The model shows sigma- and pi-bonding orbitals, and the concept of hybridisation and delocalisation. A carbon and two hydrogen atoms form the left and right sides of the simplest alkene compound - ethene. In an sp2 hybridization, one s orbital is mixed with two p orbitals to form three sp2 hybridized orbitals. Much of the production of ethene goes to polyethylene manufacture and also as a plant hormone to speed up the ripening of fruit.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP26437015
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading