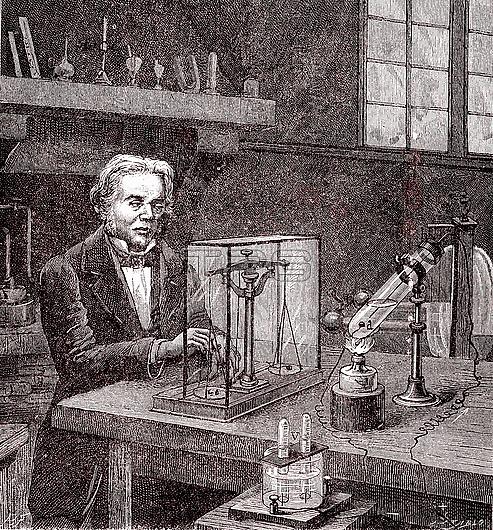
Faraday's electrolysis experiment. 19th-century illustration of British chemist and physicist Michael Faraday (1791-1867) experimenting on electrolysis in 1833. The test tube (lower right) contains two platinum electrodes dipped in molten tin chloride heated by a spirit lamp. The electrodes are connected to a battery (not seen) and a voltmeter (bottom centre). The amount of hydrogen and oxygen gas produced in the voltmeter is a measure of the amount of electricity used. Chlorine is produced at the positive electrode (wire) and tin at the negative electrode (round coil). Weighing the coil showed that the amount of tin deposited was proportional to the amount of electricity. This illustration is from 'Physique Populaire' (Emile Desbeaux, 1891).
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP26515526
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading