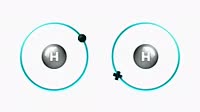
Animation showing how electron transitions within a hydrogen atom produce the Paschen series, a set of emission lines in the spectrum of hydrogen. A hydrogen atom is shown at left, opening to reveal its first eight electron shells. The shell closest to the nucleus, n=1, is the lowest energy shell, called the ground state. The atom also has other shells, n=2, n=3 and so on, at successively higher energies. The electron in a hydrogen atom can often be found in an excited state, an energy level above n=1. When it falls from the higher level back to the lower levels, it emits radiation at a characteristic frequency and wavelength, which depends on the energy difference between the shell and the ground state. As seen here, the relatively short transition between n=4 and n=3 emits radiation with a wavelength of 1875 nanometres, in the infrared part of the spectrum. The larger drop from n=8 to n=3 has the shorter wavelength (and thus higher energy) of 955nm, in the near-infrared. The complete set of transitions from excited states to n=3 form the Balmer series. As the energy levels of the shells are constant, the wavelengths are always the same. See clip K004/4145 for the Lyman series, an analogous set of emission lines in the ultraviolet, due to transitions to n=1, and K004/4146 for the Balmer series, visible light emissions due to transitions to n=2.
Details
WebID:
C01826994
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
00:40.0
Format:
QuickTime
Bit Rate:
24 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
NO
Property Release
No










 Loading
Loading