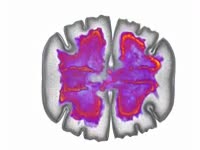
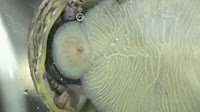
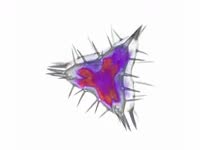
Belousov-Zhabotinsky (BZ) reaction. Footage demonstrating a Belousov-Zhabotinsky (BZ) reaction, in which bromate ions oxidise malonic acid to carbon dioxide. A solution containing bromate ions and malonic acid is mixed in a beaker using a magnetic spinner, with cerium (IV) ions as a catalyst. The reaction mixture oscillates in colour between red-brown and colourless with a time period of about 20 seconds, in two auto-catalytic processes. Bromate ions (colourless) are reduced to molecular bromine (red-brown), and then to bromide ions (colourless) in the presence of cerium ions, and malonic acid is oxidised to carbon dioxide and water. This reaction is an example of a class of reactions called Belousov-Zhabotinsky (BZ) reactions and is an example of non-equilibrium thermodynamics, and a non-linear chemical oscillator. For high-speed footage of this reaction, see clip K004/5018.
Details
WebID:
C01839994
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
00:00:20.000
Format:
QuickTime
Bit Rate:
25 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
NO
Property Release
No









 Loading
Loading