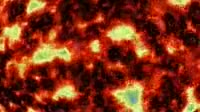
Belousov-Zhabotinsky (BZ) reaction. Footage demonstrating a Belousov-Zhabotinsky (BZ) reaction, in which bromate ions oxidise ethyl acetoacetate. A solution containing bromine and ethyl actoacetate is mixed in a beaker using a magnetic spinner and turns blue. With cerium ions as a catalyst, the reaction mixture oscillates in colour between green, blue, violet and red over a time period of about 20 seconds in two auto-catalytic processes, involving the bromate ions oxidising the ethyl acetoacetate, and oxidation of cerium III ions to cerium IV ions. The ratio of concentration of cerium (III) and cerium (IV) ions oscillates. This is an example of a class of reactions called Belousov-Zhabotinsky (BZ) reactions and is an example of non-equilibrium thermodynamics, and a non-linear chemical oscillator.
Details
WebID:
C01841387
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
00:01:16.000
Format:
QuickTime
Bit Rate:
25 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
NO
Property Release
No












 Loading
Loading