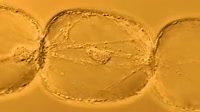
Animation of the infrared spectrum of ethanol (the alcohol in drinks) and an ethanol molecule, showing how the shape of the molecule produces the pattern of the spectrum. IR spectroscopy produces a characteristic pattern for every molecule, and this has been used in tests for alcohol, for instance in some breathalysers used to determine alcohol consumption by suspected drink drivers. Under infrared light some of the bonds linking the atoms are excited (red), and they absorb specific wavelengths of the IR energy, which makes them stretch more vigorously than usual. The O-H bond absorbs light with a wavenumber of 3391cm-1 (2.95 microns wavelength), the C-H bonds around 2961cm-1 (3.38 microns) and the C-O bond at 1102cm-1 and 1055cm-1 (9.07 and 9.48 microns).
Details
WebID:
C00725329
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
00:00:22.000
Format:
QuickTime
Bit Rate:
24 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
NO
Property Release
No












 Loading
Loading