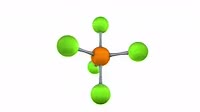
Animation of a model of a molecule of hexane (C6H14), showing the net distribution of electric charge around it. The symmetry of the linear hexane molecule leaves it with no external dipole, and it is classified as non-polar. At a macroscopic level, intermolecular attraction between opposite poles of polar molecules mean that they typically have higher melting and boiling points that non-polar compounds. Compare this situation with a molecule of ethanol, in clip K004/4337.
Details
WebID:
C01808233
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
00:00:10.000
Format:
QuickTime
Bit Rate:
24 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
NO
Property Release
No












 Loading
Loading